An overwhelming consensus on any topic is very rare these days. But many Americans, whatever their political leanings, seem to feel that the policies, communications, and actions of the public health "experts" and politicians about the COVID-19 pandemic have been confusing, sometimes contradictory, and in some cases, inconsistent with the scientific evidence.
Whether it was flip-flops on the effectiveness of masks, seemingly inane restrictions on certain activities, or baseless advocacy of ineffective drugs, the past 20 months have provided numerous reasons to question what we were told. In other cases, the absence of sufficient information has created opportunities for armchair experts to exhibit their lack of expertise.
There is a useful but misunderstood and underused mitigation tool – rapid-result antigen tests which detect pieces of the SARS-CoV-2 virus's proteins – that can slow the spread of infections. Used properly, these tests can provide a method to screen workers while a campaign is mounted to depoliticize the vaccines, as well as to encourage the administration of booster shots in vulnerable (vaccinated) individuals.
The rapid-result antigen tests are a self-administered initial screening tool for individuals to use before they venture into situations where they might come into contact with vulnerable people. These tests can identify individuals who are in their three-to-five-day period of maximum contagiousness, mostly before they are symptomatic. They generally involve putting a nasal swab and some reagent drops on a test card or test cassette that quickly displays two lines for positive or one for negative. Results are available in 10-30 minutes, in contrast to the slightly more accurate PCR tests, which measure viral RNA but whose results take days to be reported.

In simple terms, the rapid antigen test enables you to be (pretty) sure you are not at risk to others before you leave for any get-together, movie, indoor sporting event, trip to a crowded store, and so on. These tests could become crucial yet simple to use, requiring little more time and effort than brushing your teeth before going out. It would also be useful for schoolchildren or teachers exposed to a case of COVID-19, by converting "test and quarantine" while awaiting the results of the PCR test, to "test and stay" if the test is negative.
It is important to note that rapid antigen testing (or PCR testing, for that matter) is not a substitute for vaccination. As Harvard epidemiologist and immunologist Michael Mina has observed, vaccination and rapid testing work in distinct and complementary ways: Vaccination has its greatest impact on preventing serious illness — but not spread — while testing has its greatest impact as a tool to help identify those likely to spread infection to others. On Twitter, he has described his routine when visiting his elderly parents: He tests himself in his car before entering their home, and then frequently during his visit.
So, where are these tests? They are readily available free in the U.K. but have come late to the U.S. market in large numbers because they have been treated as a highly regulated medical device rather than a public health tool. Regulators have thus compared them to lab-run PCR tests, which are more accurate over the entire course of an illness – though not necessarily much better during the period of maximum contagiousness – and deemed them inferior in function. If rapid tests are used appropriately, PCR is the wrong measuring stick. The result has been suppressed demand for the rapid antigen tests, and prices remain high in the U.S., about $7-$12 each – if you can find them at all. A few companies, such as Google, make rapid tests available to employees at little or no cost. But in Europe they can cost under one dollar to the general public (although we do not know whether government subsidies are involved).
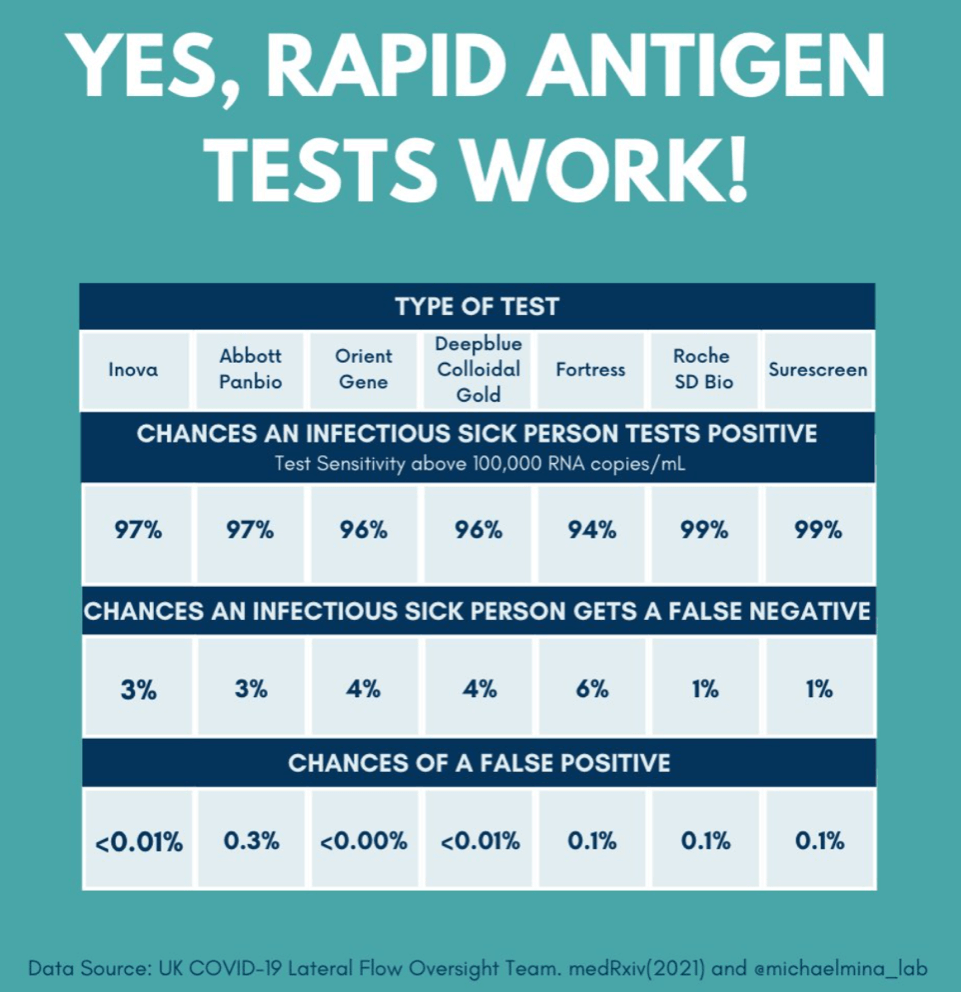
There are obvious flaws in the regulators' logic of treating these tests as devices subject to comparative evaluation. First, almost nobody electively opts to be tested if they don't have symptoms, and thereby, if infected, can become spreaders as the virus replicates during the asymptomatic period. By contrast, if the cost were minimal, people could employ these rapid tests even in the absence of symptoms as a measure of personal responsibility at little inconvenience. Second, even if a person is PCR-tested, the results take days to be reported, and an infected person can be a spreader during that time. Third, even if the rapid antigen tests miss a few positives, it's better to have most of those who self-screen and test positive remain at home than not to stop anybody at all. In other words, the rapid antigen test achieves the most important goal: limiting the number of person-days that asymptomatic but contagious people are at large, infecting others. This is discussed clearly and persuasively by Harvard Professor Mina in this video, starting about 33 minutes in.
The Biden administration seems finally to have awakened to the importance of these tests. In an effort to increase availability, the White House earlier this month allocated $1 billion to purchase millions of tests over the next year, encouraging manufacturers to ramp up production. But given the feds' enormous expenditures on the pandemic over the past 20 months, and the toll that it continues to take on the nation's health and economy, that is very little, very late.
Henry I. Miller, a physician and molecular biologist, is a Senior Fellow at the Pacific Research Institute. Find Henry on Twitter @henryimiller
Andrew I. Fillat spent his career in technology venture capital and information technology companies. He is also the co-inventor of relational databases.
Henry and Andy were undergraduates together at M.I.T.

